Organic Chemistry Carbon & Its Compound
Organic Chemistry Carbon & Its Compound – Important For Railway ALP/Technician/Group D,SSC And CDS Exams
1. Bonding in Carbon
Carbon form covalent bonds.
Formation of covalent bond : Covalent bond formation involves sharing of electrons
between bonding atoms which may be either same or different.
Covalency : The number of electrons contributed by an atom for sharing is known as its
covalency.
Characteristics of covalent compounds :
(i) These compounds are molecular in nature (i.e. they exist as single molecules)
(ii) These are insoluble in water and soluble in benzene, kerosene and petrol etc.
(iii) These compounds are poor conductor of electricity.
2. Allotropy in Carbon
The property due to which an element exists in two or more forms, which differ in their
physical and some ofthe chemical properties is known as “Allotropy” and the various forms are
called “Allotropes”.
Carbon exists in two allotropic form (i) crystalline (ii) amorphous. The crystalline forms
are diamond and graphite whereas the amorphous forms are coal, charcoal, lamp black
etc.
Fullerenes form another class of carbon allotropes. The first one to be identified was C-
60, which has carbon atoms arranged in the shape of a football.
3. Unique Nature of Carbon
Catenation : The property of elements to form long chains or rings by self linking of their
own atoms- through covalent bonds is called catenation. The extent of catenation depends upon
the strength of the bonds between the atoms involved in catenation.
4. Saturated and Unsaturated Carbon Compounds
In saturated compounds the valencies of all the carbon atoms are satisfied by single
bonds between them.
While in the unsaturated compounds, the valencies of all the carbon atoms are not satisfied by
single bonds, thus in order to satisfy their valencies, they form double or triple bond between
the carbon atoms.
5. Straight chain compounds : The compounds which contain straight chain of carbon
atoms e.g. normal butane (C4H10), normal pentane (5H12) etc.
6. Branched chain compounds : Those compounds which are branched.
e.g. iso-butane (C4H10), isopentane (C5H12), neopentane (C5H12) etc.
7. Closed chain compounds or Ring compounds :
Cyclic compounds are called closed chain or ring compounds e.g. cyclohexane
(C6H12), cyclopentane (C5H10), cyclobutane (C4H8), cyclopropane (C3H6) etc.
8. Hydrocarbons
All those compounds which contain just carbon and hydrogen are called hydrocarbons.
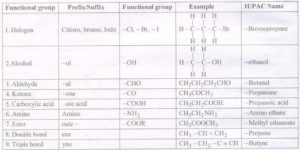
9. Functional Group
The atom or group of atoms which determine the properties of a compound is known
as functional group. e.g. —OH (alcohol), —CHO (aldehyde), > C = C < (alkene), — C—C —
(alkyne) etc.
10. Homologous Series
A series of compounds in which the same functional group substitutes hydrogen in a carbon
chain is called a homologous series.
e.g. CH3C1 and C2H5C1 differ by a —CH2 unit.
11. Nomenclature
Chemists developed a set of rules, for naming organic compounds based on their
structures which is known as IUPAC rules.
The IUPAC name of an organic compounds consists of three parts.
Prefix – word root – Suffix
Word Root : A word root indicates the nature of basic carbon skeleton.
In case a functional group is present, it is indicated in the name of the compound with either as
a prefix or as a suffix.
While adding the suffix to the word root the terminal „e‟ of carbon chain is removed If the
carbon chain is unsaturated then the final `ane‟ in the name of the carbon chain is substituted
by „en& or yne‟ respectively for double and triple bonds.